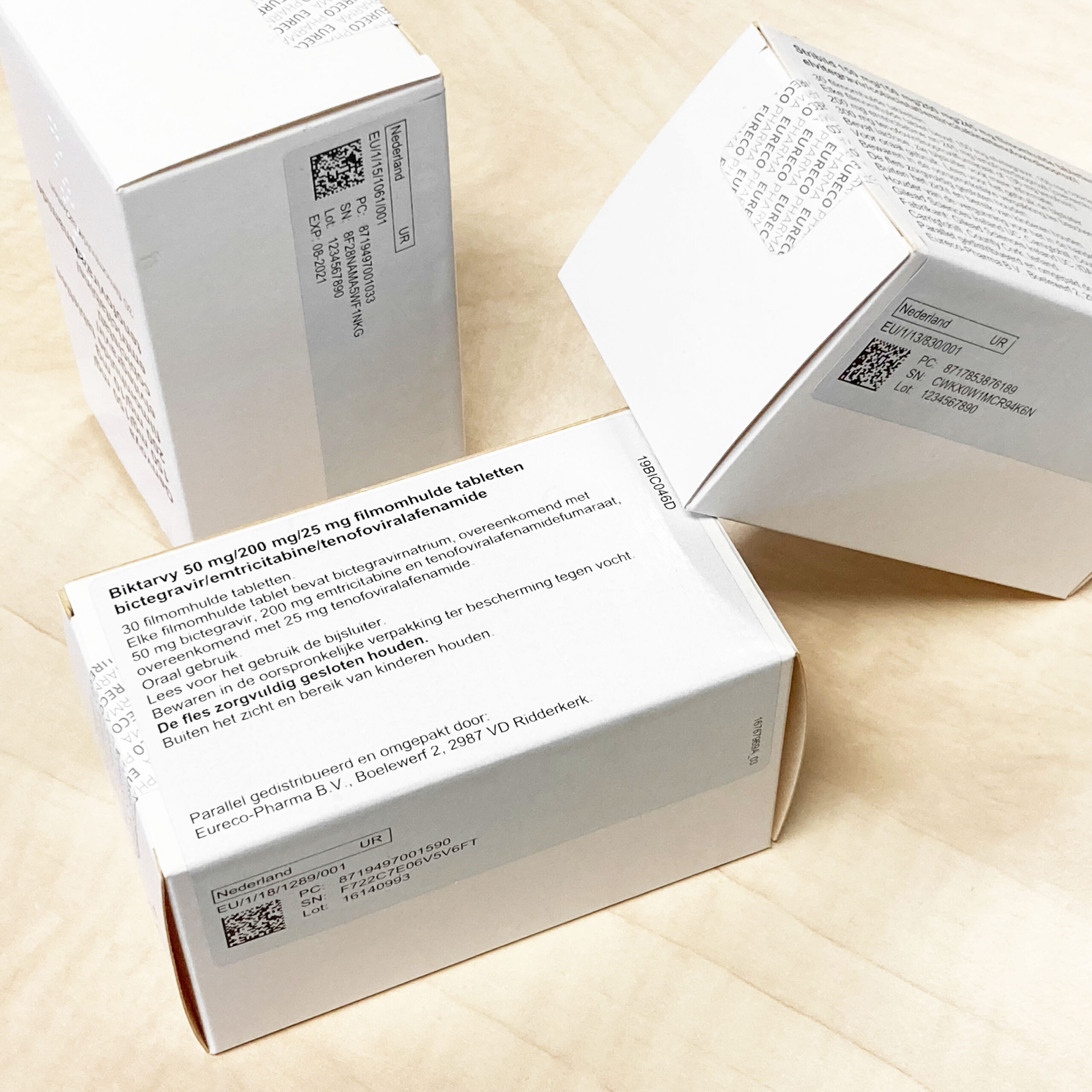
Data integration on labels and packaging
Every prescription medicine dispatched from our facilities is furnished with safety provisions in compliance with the European guidelines of the FMD (2016/161/EU). These encompass an Anti-Tampering Device (ATD) as an anti-tampering measure and a Unique Identifier (UI) comprising a distinctive serial number. The assurance of this data is, of course, done in accordance with GMP. And in order to continuously check and register all this as well as possible, a very user-friendly interface is used.
Do you have questionsabout this innovation,please contact us
Subscribe to ournewsletter
Please sign up for our newsletter. Stay informed about the latest updates and our medicines, services, and innovations. After signing up, we will send a confirmation of your registration upon approval..
Subscribe to our newsletter
Please sign up for our newsletter. Stay informed about the latest updates and our medicines, services, and innovations. After signing up, we will send a confirmation of your registration upon approval..
Subscribe to our newsletter
Please sign up for our newsletter. Stay informed about the latest updates and our medicines, services, and innovations. After signing up, we will send a confirmation of your registration upon approval..